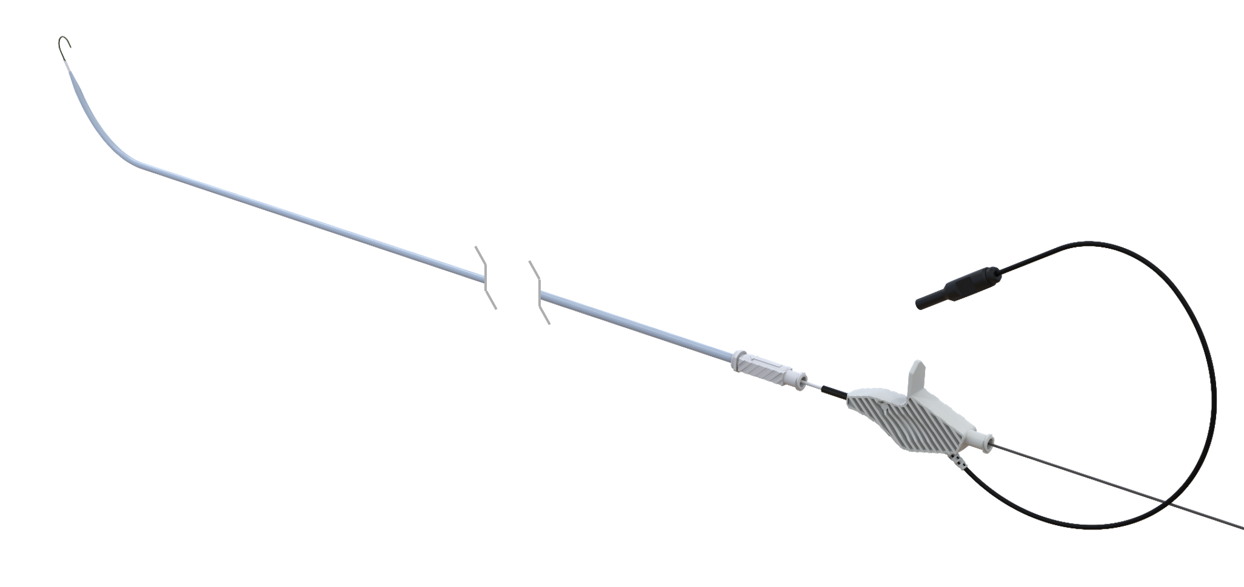
CrossWise RF Transseptal Access System
Control. Choice. Confidence.
CrossWise is a next generation RF transseptal access system facilitating over the wire zero exchange workflow for controlled, confident crossing.
Control. Choice. Confidence.
Control.
Designed for stiff rail support to aid in secure crossing and catheter delivery. Shapeable cannula allows for repositioning and precise targeting on the Fossa Ovalis.
Choice.
Compatible with commonly used steerable or fixed sheaths. Use with the tools and techniques of your preferred workflows.
Confidence.
Dielectrically coated inner and outer lumen of the canula, designed to safely facilitate guidewire retention throughout the procedure without electrical loss.

Optimized Zero Exchange Workflow
CrossWise can be backloaded over the wire for zero exchanges and fewer steps
Product Codes & Specifications
|
Product Code |
Description |
Size |
Compatible Guide Sheath |
|
CW-1085A |
CrossWise 8.5Fr, 97cm C1 Agilis |
8.5F |
St. Jude Medical- Agilis NxT Steerable Introducer 71cm (Small, Med., Large Curl) |
|
CW-1085S |
CrossWise 8.5Fr, 71cm C1 Swartz |
8.5F |
St. Jude Medical- Swartz Braided Transseptal Guiding Introducer 63cm, SL0 and SL1 |
|
CW-1012C |
CrossWise 12Fr, 88cm C1 FlexCath |
12F |
Medtronic FlexCath Advance Steerable Sheath |
|
CW-1012W |
CrossWise 12Fr, 88cm C1 Watchman |
12F |
Boston Scientific WATCHMAN, Single Curve |
|
CW-1001 |
CrossWise Multi-Use RF Adapter cable |
NA |
NA |
Ordering Information
|
Will this product replace a current product? |
Yes, this technology can replace transseptal access devices like BRK needles and alternative RF-based wire/needle access devices |
|
Is this item/technology on contract with GPOs and/or IDNs and pricing terms? |
In process, speak with your CIRCA representative for contract status and specifics |
|
Evaluation terms? |
Please speak with your CIRCA representative for specific needs |
|
Lowest UOM? |
1 each |
|
Minimum order quantity? |
5 eaches |
|
Is this a dated product? |
Yes, with a 2-year shelf life from date of manufacture |
|
Mode of transportation? |
Standard delivery FedEx ground |
|
Lead time in working days? |
3-5 days |
|
Method of supply? |
Direct purchase, no consignment |
|
What department(s) will use or be affected? |
Cardiac electrophysiology and cardiac catheterization lab |
|
Will there be additional implementation costs? Installation, education, space? |
No; in-servicing of physicians and staff by a trained CIRCA representative is required prior to use. |
|
Will this product require evaluation by any other departments? |
Epidemiology/Infection Control? No : Safety and Security? No Bio-Engineering/Maintenance? No : Pathology/Labs? No |
|
Does this item contain natural rubber latex? |
Products and packaging are not made with natural rubber latex. |
Key Benefits
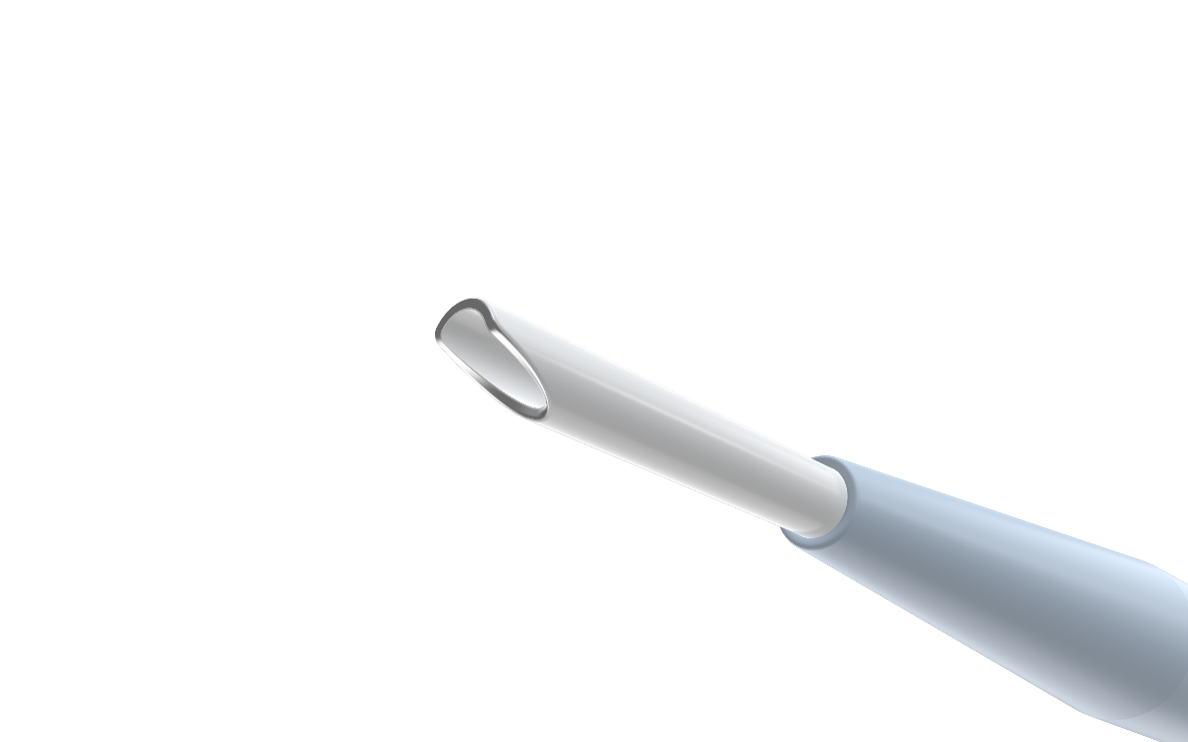
Visual proximal indicator sleeve identifies when the cannula tip is outside the dilator, allowing user to control advancement while crossing.
Radiopaque cannula allows for easy visualization on fluoroscopy
Guidewire flexibility allows standard 0.032” choice to be backloaded and ready to deliver upon crossing. No proprietary capital equipment required for use. Compatible with commercially available electrosurgical pencil.
Patented focal force electrode delivering RF energy only at the tip. Bevel designed with atraumatic shape for smoother controlled crossing
CrossWise Resources
Accessories

The CrossWise Multi-Use RF Adapter Cable connects the CrossWise RF Transseptal Access System to commercially available electrosurgical pencils.
Ready to Trial CrossWise?
Click the button below to request an evaluation of the CrossWise RF Transseptal Access System.
Related Products
CIRCA Esophageal Temperature Monitoring System
The CIRCA Temperature Monitoring System™ is designed to provide fast response to hot and cold temperature changes.
LEARN MORE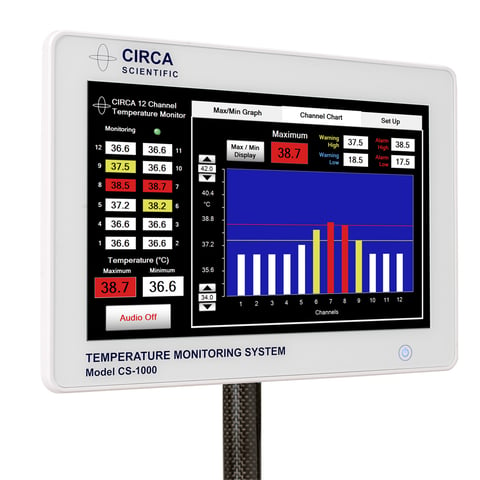
S-CATH™ Esophageal Temperature Probe
The S-CATH deploys an array of 12 coated, rapid response temperature sensors on a soft, flexible, “S” shape
LEARN MORE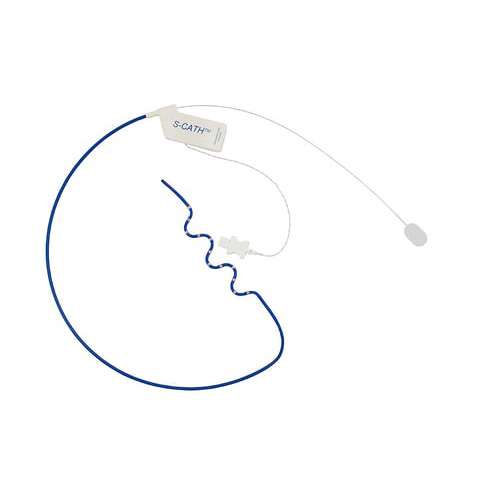
S-CATH™ M Esophageal Temperature Probe

MATRIX12 M™ Esophageal Temperature Probe
The MATRIX12 M provides with its unique GaplessGrid technology provides unprecedented speed, comprehensive coverage, and enhanced visualization on 3D cardiac mapping systems.
LEARN MORE
Regulatory Information
Indications for Use
The CrossWise™ RF Transseptal Cannula and accessories are used to create an atrial septal defect in the heart. Secondary indications include infusing solutions including heparinized saline and mixtures of 50% contrast media and 50% saline.
Regulatory Clearances and Standards
FDA 510(k) Clearance